is h2co polar Polar h2co non molecule formaldehyde
As professionals in the field of chemistry, it is important that we have a deep understanding of the polarity of various molecules, including H2CO. H2CO, also known as formaldehyde, is a simple organic compound that is widely used in the production of many industrial and consumer products. Its molecular structure consists of three atoms: carbon, oxygen, and hydrogen. The question arises, is H2CO polar or non-polar? In order to answer this, we need to look at the electronegativity of each atom present in the molecule. Electronegativity is the measure of an atom’s ability to attract electrons towards itself in a covalent bond. In the case of H2CO, the carbon atom is partially positive, while the oxygen atom is partially negative. This is due to the difference in electronegativity between the two atoms. The oxygen atom is much more electronegative than the carbon atom, which means it attracts the shared electrons towards itself and becomes partially negative. The hydrogen atoms, on the other hand, have a partial positive charge due to being less electronegative than carbon. As a result of this uneven distribution of charges, H2CO is a polar molecule. The molecule has a dipole moment, which means it has a positive end and a negative end. The dipole moment is a measure of the magnitude of this difference in charge. The implications of H2CO being polar are significant. It affects the way the molecule interacts with other molecules in its environment. Polar molecules tend to interact more strongly with other polar molecules than non-polar molecules do. This is due to the presence of positive and negative charges that can attract each other. In practical terms, this means that H2CO will dissolve more readily in polar solvents than non-polar solvents. It will also interact more strongly with other polar molecules, such as water. This has important consequences in terms of its use in various industrial and consumer applications. For example, H2CO is used in the production of resins and plastics, where it interacts strongly with other polar molecules in order to form strong bonds. In conclusion, the polarity of H2CO has significant implications for its behavior and interactions with other molecules. As professionals in the field of chemistry, it is important that we understand the polar nature of H2CO in order to make informed decisions regarding its various applications.
If you are searching about Is H2CO polar or nonpolar you’ve visit to the right page. We have 5 Pics about Is H2CO polar or nonpolar like ShowMe - molecular polarity of H2cO, Is H2CO polar or nonpolar and also Is H2CO Polar of Non-Polar? - YouTube. Read more:
Is H2CO Polar Or Nonpolar
 www.bengislife.compolar h2co nonpolar non
www.bengislife.compolar h2co nonpolar non
H2CO Lewis Structure, Molecular Geometry, Hybridization, And MO Diagram - Techiescientist
 techiescientist.comformaldehyde ch2o h2co hcho polar nonpolar sigma hybridization methanol techiescientist methanal ethylhexylglycerin reagent acs stabilizer polymerization sds wt trigonal chemical
techiescientist.comformaldehyde ch2o h2co hcho polar nonpolar sigma hybridization methanol techiescientist methanal ethylhexylglycerin reagent acs stabilizer polymerization sds wt trigonal chemical
Is H2CO Polar Of Non-Polar? - YouTube
 www.youtube.compolar h2co
www.youtube.compolar h2co
ShowMe - Molecular Polarity Of H2cO
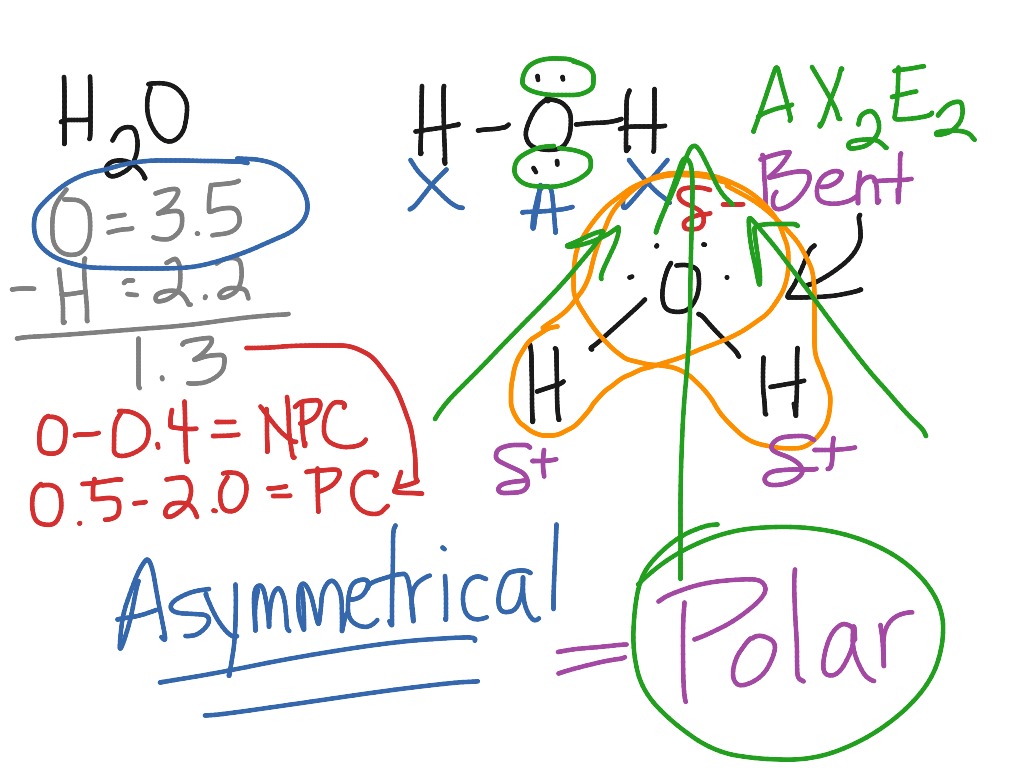 www.showme.compolarity h2o h2co molecular
www.showme.compolarity h2o h2co molecular
Is H2CO A Polar Or Non-polar Molecule? - Quora
www.quora.compolar h2co non molecule formaldehyde
Is h2co a polar or non-polar molecule?. Polar h2co nonpolar non. H2co lewis structure, molecular geometry, hybridization, and mo diagram